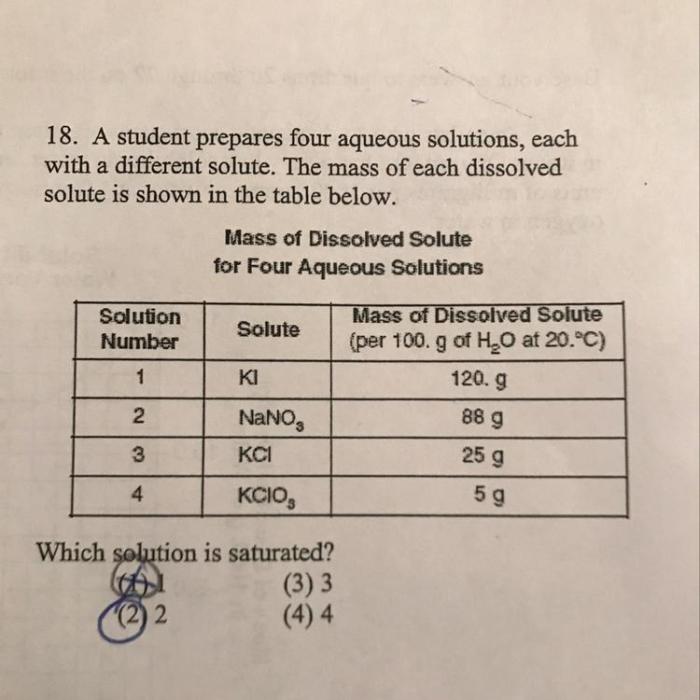
A student prepares four aqueous solutions – Delving into the realm of chemistry, this comprehensive guide explores the meticulous process of preparing aqueous solutions. Aqueous solutions, the cornerstone of numerous chemical reactions and scientific investigations, hold immense significance in various fields. This guide empowers students with the knowledge and techniques required to prepare aqueous solutions with precision and confidence, fostering a deeper understanding of their applications and the intricate world of chemistry.
Introduction
An aqueous solution is a solution in which water is the solvent. Water is an excellent solvent because it is polar and can dissolve many different types of substances. Aqueous solutions are used in a wide variety of applications, including chemistry, biology, and medicine.
Preparing aqueous solutions is a basic laboratory skill. The procedure is relatively simple, but there are a few important things to keep in mind.
Materials and Equipment
To prepare an aqueous solution, you will need the following materials and equipment:
- Solute
- Solvent (water)
- Graduated cylinder
- Beaker
- Stirring rod
The solute is the substance that is being dissolved in the water. The solvent is the liquid that dissolves the solute. The graduated cylinder is used to measure the volume of the solvent. The beaker is used to hold the solution.
The stirring rod is used to stir the solution.
Procedure
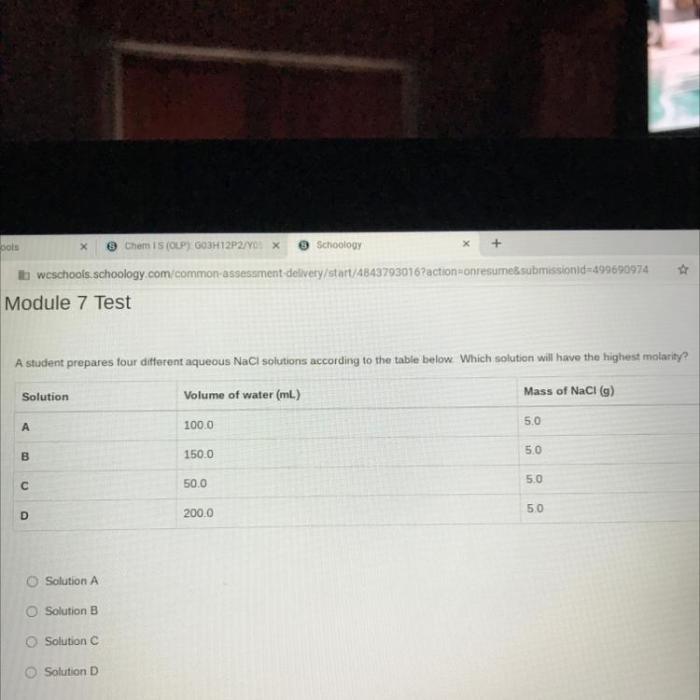
To prepare an aqueous solution, follow these steps:
- Measure the desired volume of water into a graduated cylinder.
- Pour the water into a beaker.
- Add the solute to the water.
- Stir the solution until the solute is completely dissolved.
Once the solution is prepared, it is important to store it properly. Aqueous solutions can be stored in a sealed container at room temperature.
Calculations
The concentration of an aqueous solution is a measure of the amount of solute that is dissolved in the water. The concentration can be expressed in a variety of units, including molarity, molality, and weight percent.
The following formula can be used to calculate the concentration of an aqueous solution:
Concentration = (moles of solute) / (volume of solution in liters)
For example, if you dissolve 0.1 moles of NaCl in 1 liter of water, the concentration of the solution would be 0.1 M.
Applications: A Student Prepares Four Aqueous Solutions

Aqueous solutions are used in a wide variety of applications, including:
- Chemistry: Aqueous solutions are used in a variety of chemical reactions. For example, aqueous solutions of acids and bases are used to neutralize each other.
- Biology: Aqueous solutions are used to transport nutrients and waste products in living organisms. For example, blood is an aqueous solution that transports oxygen and nutrients to cells.
- Medicine: Aqueous solutions are used to deliver drugs to the body. For example, saline is an aqueous solution that is used to hydrate patients.
Troubleshooting
If you are having trouble preparing an aqueous solution, there are a few things that you can check:
- Make sure that you are using the correct solvent. Water is the most common solvent, but some solutes require a different solvent.
- Make sure that you are using the correct amount of solute. Too much solute can make the solution too concentrated, and too little solute can make the solution too dilute.
- Make sure that you are stirring the solution thoroughly. Stirring helps to dissolve the solute and create a uniform solution.
If you are still having trouble, you can consult with a laboratory instructor or a chemist.
Questions and Answers
What is the purpose of preparing aqueous solutions?
Aqueous solutions are essential in chemistry as they allow for the precise control of reactant concentrations, enabling the study of chemical reactions and their kinetics. They also serve as the basis for various analytical techniques and are widely used in fields such as biology, medicine, and environmental science.
How do I calculate the mass of solute required to prepare an aqueous solution of a specific concentration?
To calculate the mass of solute required, use the formula: mass of solute = concentration × volume of solution × molar mass of solute. For example, to prepare 100 mL of a 0.1 M NaCl solution, you would need 0.1 mol/L × 0.1 L × 58.44 g/mol = 0.5844 g of NaCl.
What safety precautions should I observe when preparing aqueous solutions?
Always wear appropriate personal protective equipment, including gloves, safety glasses, and a lab coat. Handle chemicals with care, avoid direct contact with skin and eyes, and work in a well-ventilated area. Dispose of chemicals and solutions properly according to established protocols.