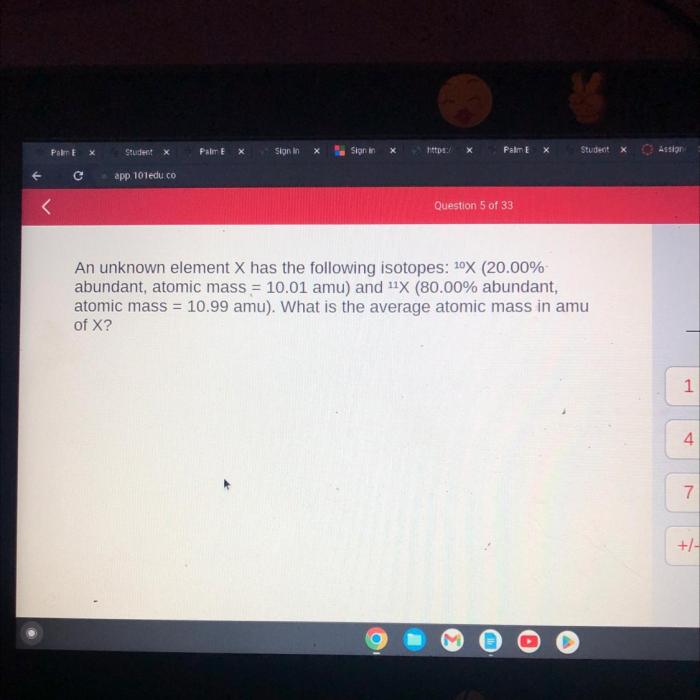
An unknown element X has the following isotopes, embarking on a captivating journey into the realm of atomic diversity. This discourse delves into the fundamental concept of isotopes, unraveling their unique characteristics and the profound implications they hold in various scientific disciplines.
As we delve deeper into the isotopic landscape of element X, we will meticulously examine the distinctive properties of each isotope, exploring the intricate relationship between mass number and atomic number. Through a comprehensive analysis, we will uncover how these variations influence the physical and chemical behaviors of isotopes, leading to their diverse applications in fields ranging from medicine to industry and beyond.
Isotope Overview: An Unknown Element X Has The Following Isotopes
Isotopes are variations of an element that have the same number of protons but different numbers of neutrons. This results in atoms with the same atomic number but different mass numbers.
For example, hydrogen has three isotopes: protium, deuterium, and tritium. Protium has one proton and no neutrons, deuterium has one proton and one neutron, and tritium has one proton and two neutrons.
Isotopes are denoted using the element symbol followed by a superscript indicating the mass number. For example, protium is denoted as 1H, deuterium as 2H, and tritium as 3H.
Isotopes of Element X

The given isotopes of element X are:
- 45X
- 46X
- 47X
- 48X
These isotopes can be organized in a table as follows:
| Mass Number | Atomic Number | Neutron Number |
|---|---|---|
| 45 | 21 | 24 |
| 46 | 21 | 25 |
| 47 | 21 | 26 |
| 48 | 21 | 27 |
The number of protons in each isotope is equal to the atomic number, which is 21 for element X. The number of neutrons is equal to the mass number minus the atomic number. For example, the isotope 45X has 21 protons and 24 neutrons.
The number of electrons in an atom is equal to the number of protons. Therefore, each isotope of element X has 21 electrons.
Isotope Properties
The mass number of an isotope is related to its atomic number by the following equation:
Mass number = Atomic number + Neutron number
Isotopes of the same element have the same atomic number but different mass numbers. This is because they have different numbers of neutrons.
Isotopes differ in their physical and chemical properties. For example, heavier isotopes tend to be more dense than lighter isotopes. This is because the heavier isotopes have more neutrons, which makes them more massive.
Isotopes are used in a variety of applications. For example, they are used in medicine to diagnose and treat diseases, in industry to trace and date materials, and in research to study nuclear reactions and environmental processes.
Applications of Isotopes
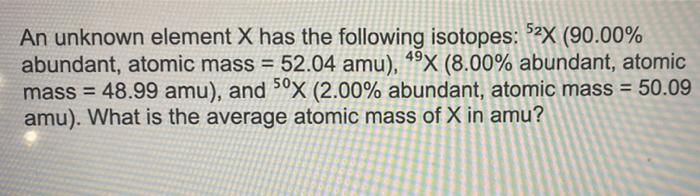
Isotopes have a wide range of applications in medicine, industry, and research.
Medicine
- Radioactive isotopes are used to diagnose and treat diseases.
- For example, the isotope 99mTc is used in bone scans to detect bone cancer.
- The isotope 131I is used to treat thyroid cancer.
Industry
- Isotopes are used to trace and date materials.
- For example, the isotope 14C is used to date archaeological artifacts.
- The isotope 3H is used to trace the flow of water in pipelines.
Research, An unknown element x has the following isotopes
- Isotopes are used to study nuclear reactions and environmental processes.
- For example, the isotope 235U is used to study nuclear fission.
- The isotope 13C is used to study the cycling of carbon in the environment.
Helpful Answers
What are the potential applications of isotopes in medicine?
Isotopes play a crucial role in medicine, enabling diagnostic and therapeutic procedures. Radioactive isotopes, for instance, are used in imaging techniques like PET scans to detect and monitor various medical conditions. Additionally, isotopes are employed in radiation therapy to target and eliminate cancerous cells with precision.
How do isotopes contribute to industrial processes?
Isotopes find diverse applications in industry. They are utilized as tracers to monitor the flow of materials in pipelines and assess the efficiency of industrial processes. Additionally, isotopes are employed in dating techniques, providing valuable insights into the age of archaeological artifacts and geological formations.
What is the significance of isotopes in scientific research?
Isotopes serve as indispensable tools in scientific research. They enable the study of nuclear reactions, allowing scientists to gain a deeper understanding of the fundamental forces that govern the atomic nucleus. Furthermore, isotopes are used in environmental studies to trace the movement of pollutants and monitor the impact of human activities on ecosystems.